质量提高和下降都是不行的。
We note that while improvement of product quality is always desirable and encouraged, if the results of comparability studies indicate an improved product quality suggesting a significant benefit in effectiveness and/or safety, the pre- and post-change products may be different products and, therefore, not comparable.
…… in some cases analytical studies alone may not be sufficient to reach a conclusion regarding comparability. In such cases, additional data from nonclinical studies may help to support comparability. Otherwise, additional clinical studies may be warranted.
解读:质量的显著提升也被认为是不可比的,个人理解是无论是效价提升还是安全性提升,变更后的产品和之前的临床数据无法画等号,这就缺乏数据支持。这种情况怎么办?做非临床桥接,乃至临床的桥接。
指南——Manufacturing Changes and Comparability for Human CGT Products
质量下降更不用说了。
附上一张图片供参考,是业内会议上的分享,找不到出处了:
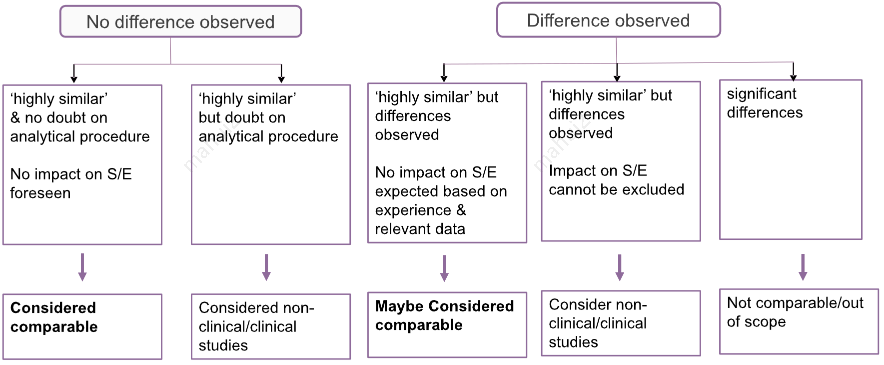
1、Comparability between the pre-change and post-change products is generally demonstrated by evidence that the change does not adversely affect product quality for the licensed (21 CFR 601.12(a)(2)) or investigational product. However, if the change is intended to improve product quality, such that there is a significant benefit in effectiveness and/or safety, then the post-change product may be considered a different product, and therefore not comparable to the pre-change product. We recommend that you seek FDA advice (section VII of this guidance)
变更前和变更后产品之间的可比性通常通过证据表明变更不会对许可(21 CFR 601.12(a)(2))或研究用产品的产品质量产生不利影响。但是,如果变更是为了提高产品质量,从而在有效性和/或安全性方面有显着的获益,那么变更后的产品可能被认为是不同的产品,因此与变更前的产品不具有可比性。当计划重大生产变更以及设计可比性研究方案时,我们建议您寻求FDA建议(本指南第VII节)。来源:FDA Manufacturing Changes and Comparability for Human Cellular and Gene Therapy Products
2、“开展变更可比性研究是生物制品上市后药学变更评价的基础和成功的关键。”《已上市生物制品药学变更研究技术指导原则(试行)》。发起变更,如果变更前后不可比,无论是质量提高亦或者降低,都无法按照药学变更的审批流程开展
这{{threadTextType}}正{{isAdminText}}
为帮助审核人员更快处理,请填写举报原因:
为帮助审核人员更快处理,请填写举报原因:

1、Comparability between the pre-change and post-change products is generally demonstrated by evidence that the change does not adversely affect product quality for the licensed (21 CFR 601.12(a)(2)) or investigational product. However, if the change is intended to improve product quality, such that there is a significant benefit in effectiveness and/or safety, then the post-change product may be considered a different product, and therefore not comparable to the pre-change product. We recommend that you seek FDA advice (section VII of this guidance)
变更前和变更后产品之间的可比性通常通过证据表明变更不会对许可(21 CFR 601.12(a)(2))或研究用产品的产品质量产生不利影响。但是,如果变更是为了提高产品质量,从而在有效性和/或安全性方面有显着的获益,那么变更后的产品可能被认为是不同的产品,因此与变更前的产品不具有可比性。当计划重大生产变更以及设计可比性研究方案时,我们建议您寻求FDA建议(本指南第VII节)。来源:FDA Manufacturing Changes and Comparability for Human Cellular and Gene Therapy Products
2、“开展变更可比性研究是生物制品上市后药学变更评价的基础和成功的关键。”《已上市生物制品药学变更研究技术指导原则(试行)》。发起变更,如果变更前后不可比,无论是质量提高亦或者降低,都无法按照药学变更的审批流程开展。